The rate of this step and therefore the rate of the overall substitution reaction depends on the activation energy for the process in which the bond between the carbon and the leaving group breaks and a carbocation forms.
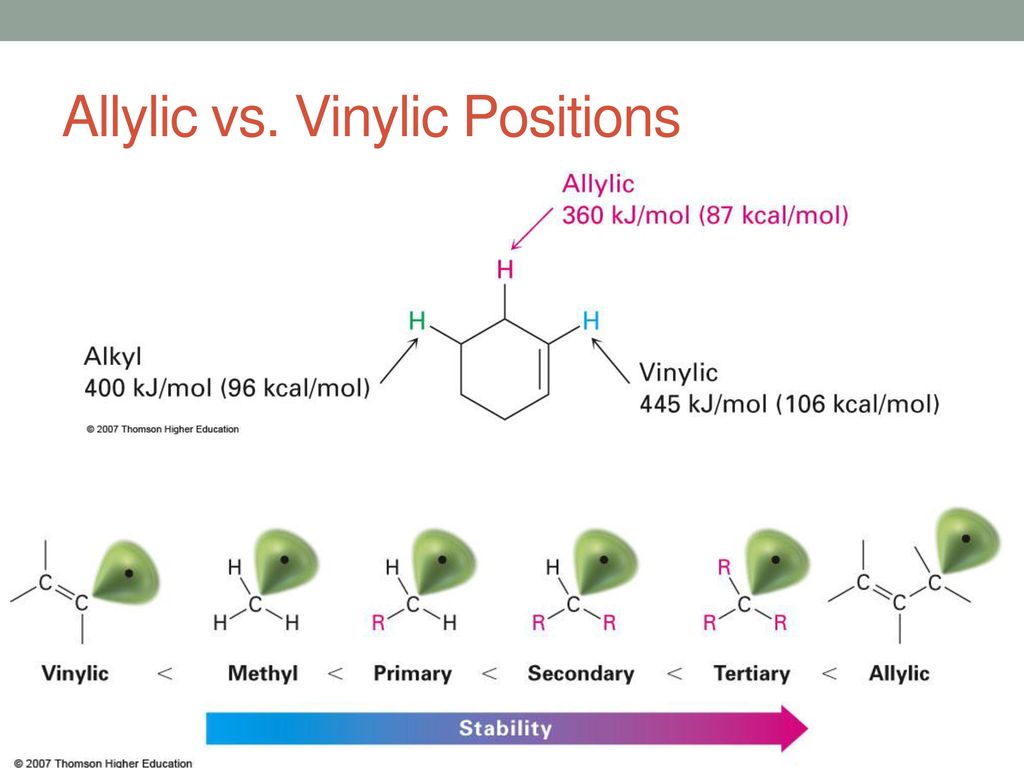
Ch bond strength tertiary vs vinylic.
Terminal alkynes that is to say those where the triple bond is at the end of a carbon chain have c h bonds involving the sp carbon the carbon that forms part of the triple bond.
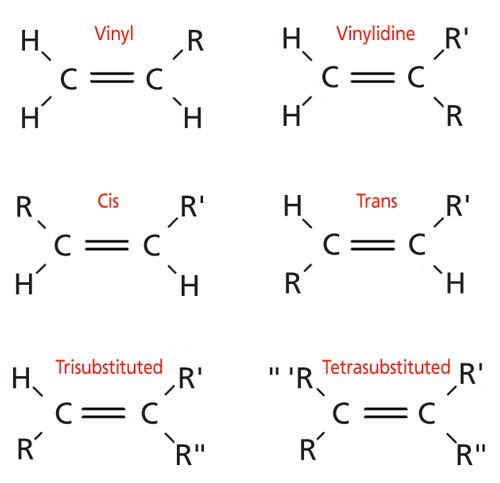
The general formula for vinyl group is r ch ch 2 in which both carbon atoms are bonded with double bond and r is attached at vinylic position.
The first of these is covalent bond strength.
Remarkably this is the strongest common single bond to carbon being roughly 30 kcal mole stronger than a carbon carbon bond and about 15 kcal mole stronger than a carbon hydrogen bond.
The double bonded carbon atoms can be classified as vinylic and allylic carbon atoms.
This completes both of their outer shells making them stable.
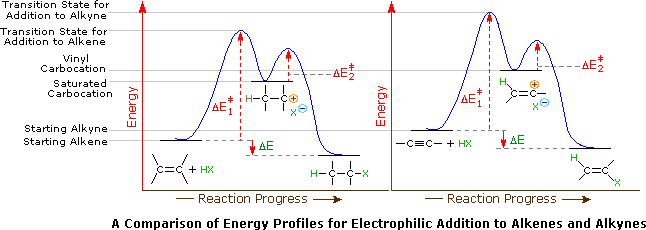
The vinyl cation is a carbocation with the positive charge on an alkene carbon.
The low polarity of the triple bond associated with those alkynes.
We know that the rate limiting step of an s n 1 reaction is the first step formation of the this carbocation intermediate.
This bond is a covalent bond meaning that carbon shares its outer valence electrons with up to four hydrogens.
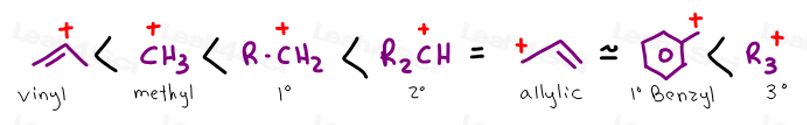
Stability of carbocation intermediates.
Its empirical formula is c 2 h 3 more generally a vinylic cation is any disubstituted trivalent carbon where the carbon bearing the positive charge is part of a double bond and is sp hybridized in the chemical literature substituted vinylic cations are often referred to as vinyl cations and understood to.
Since both carbon atoms form a double covalent bond so both are sp 2 hybridized.
The allylic carbon atom is more reactive than normal.
R r and r are alkyl groups and may be the same or different.
Free radicals are stabilized by adjacent atoms with lone pairs.
The strongest of the carbon halogen covalent bonds is that to fluorine.
A tertiary carbocation has the general formula shown in the box.
The key difference between allylic and vinylic carbon is that allylic carbon is the carbon.
Therefore they may also show a sharp weak band at about 3300 cm 1 corresponding to the c h stretch.
Key difference allylic vs vinylic carbons functional groups are very important in understanding the different physical and chemical properties of organic molecules the terms allylic and vinyl carbons indicate whether the carbon atom is bonded directly or indirectly to a double bond in a molecule.
The stability of the various carbocations.
This doubly allylic c h bond is even weaker reflecting the fact that a greater number of resonance forms are available for the radical species.
The allylic position is also like a.
Although not directly comparable look at the c h bond strength when it is adjacent to two alkenes 76 kcal mol.
Carbon hydrogen bonds have a bond length of about 1 09 å 1 09 10 10 m and a bond.